The electron configuration of a Phosphorus atom in its ground state is 1s2 2s2 2p6 3s2 3p3 but when it is in an excited state the electrons from 3s orbital get unpaired. Experts are tested by Chegg as specialists in their subject area.

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Draw the Lewis structure of SF2 showing all lone pairs.
. X represents the bonded atoms as we know silicon is making four bonds with chlorine atoms. The actual molecule is an average of structures 2 and 3 which are called resonance structures. Phosphorus pentachloride is a greenish-yellow crystalline solid with an irritating odor.
See the answer See the answer done loading. Identify the molecular geometry of XeCl4. For the Lewis structure for PCl5 you should take formal charges into account to find the best Lewis structure for the molecule.
Sp sp2 sp3 sp3d sp3d2 An SF2 molecule is polar nonpolar. 3D shape trigonal bi pyramidal. Lewis structures also known as Lewis dot formulas Lewis dot structures electron dot structures or Lewis electron dot structures are diagrams that show the bonding between atoms of a molecule as well as the lone pairs of electrons that may exist in the molecule.
What is the hybridization of the central atom. In solid state it will be in charged form. A step-by-step explanation of how to draw the PCl4- Lewis Dot StructureFor the PCl4- structure use the periodic table to find the total number of valence el.
Carbon contains four valence electrons resulting in. How do you draw the Lewis Structure for PCl5. Oxygen contains 6 valence electrons which form 2 lone pairs.
We have a total of 40 valence electrons for the AsF5 Lewis structure. Since it is bonded to only one carbon atom it must form a double bond. Cl has 5 x 7 35.
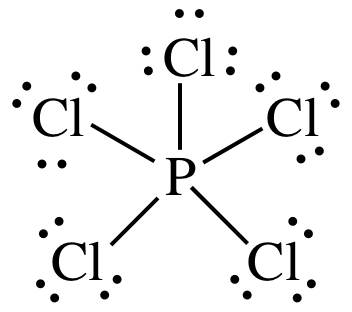
There are a total of 40 valence electrons in the PCl5 Lewis structure. Total valence shell electron pairs are 5. In the Lewis structure for PCl 5 there are a total of 40 valence electrons.
Each Cl has 3 lone pairs but P has no lone pairs. Then count 10 more for the 5 bonds for each P-Cl and that is the 40 electrons available. PCl 5 lewis structure.
Draw the lewis structure of the following molecule. Drawing the Lewis Structure for PCl 5. Therefore X 4.
We review their content and use your feedback to keep the quality high. Include all lone pairs. Then well go around the outside and complete the octets for the Fluorine.
PCl 5 is similar to PBr 5 and PF 5. The PCl5 structure has 2 different kinds of P-Cl bonds. Find the total number of valence electrons in a molecule- Adding up the valence electrons of all the atoms in a molecule is the first step.
Questions Answers Homework Help How do you draw the Lewis Structure for PCl5. Hence the Hybridization is determined by counting the number of bonds made and the lone pair. The central atom of this molecule is carbon.
You can see there is no lone pairs on phosphorus atom in PCl 5 as PCl 3. There is a lone pair on center. Draw the Lewis structures for TeCl 4 ICl 5 PCl 5 KrCl 4 and XeCl 2.
Well put chemical bonds between the Arsenic and the Fluorine atoms. The formula to find the Hybridization is as follows-H 12VM-CA H Hybridization V Number of Valence electrons. There are no lone pairs in the Lewis Structure of PF 5 and there are five single bonds between Phosphorus and Fluorine atoms.
As we know phosphorus belongs to the 3 rd period in the modern periodic table. PCl 5 Hybridization. One s orbital three.
There are a total of 40 valence electrons in the PCl5 Lewis structure. While electron geometry does include lone pairs. Count the 6 around each Cl and that accounts for 30 electrons.
There are five half-filled orbitals. Choose a central atom and draw a skeletal structure- Sketch a skeletal of the molecule with only single bonds. Simply P at the center and 5 chlorines around it.
The Lewis electron dot structures of a few molecules are illustrated in this subsection. A represents the central atom so as per the SiCl4 lewis structure silicon is the central atom. Lewis Structure of CO2.
Draw the Lewis structure of XeC14. There are 2 electrons in each chemical bond so weve used 2 4 6 8 10. Total e 40.
In the PCl 5 Lewis structure Phosphorus P is the least electronegative so it goes in the center. Also there is a high chance of PCl5 formation in this process. If you can do those Lewis structures PCl 5 will be easy.
Remember when you draw the Lewis structure for PCl5 that Phosphorous P is in Period 3 on the Periodic table. Now the central atom is generally the least electronegative. Then well put 5 Fluorines around the Arsenic.
Who are the experts. This means that it can hold more than 8 valence electrons. Dec 6 2007.
The Lewis formalism used for the H Write the Lewis structure for each molecule. Drawing the Lewis structure of PCl5. We can refer to the periodic table for this.
By Guest14548277 12 years 3 months ago. This means that it can hold more than 8 valence electrons. Now lets see the proper steps to draw a lewis structure-1.
Count the number of valence electrons in a PCl5 molecule. Remember when you draw the Lewis structure for PCl5 that Phosphorous P is in Period 3 on the Periodic table. PCl₅ can exist in both solid and gaseous states but is generally found in gaseous states.
P has 5 e. So to avoid that continuous removal of phosphorus trichloride is mandatory. To draw the lewis structure first of all we need to sum up the valence electrons of all the atoms.
The draw-the-lewis-structure-for-ch3no2-and-include-all-lone-pairs-and-electrons have 2021-02-25 184403 and PT7M41S. The things required to form the structure-. All the Phosphorus-Chlorine equatorial bonds make 90 degrees and 120 degrees bond angles two.
In this tutorial we will learn how to draw the lewis structure of PCl 5 step by step with all theories. In this lewis structure of PCl 5 center phosphorus atom has made five single bonds with five chlorine atoms. Compound PCl₅ comprises of phosphorus and chlorine in the ratio of P.
N represents the lone pair on the central atom silicon atom has zero lone pair on it. PCl3 has a bond angle of 103 degrees. It is decomposed by water to form hydrochloric and.
Five pairs will be used in the chemical bonds between the P and Cl. This includes the lone pair in the s orbital 3 sigma bonds in the p orbital and 2 sigma bonds in the p orbital making it Sp3d2 hybridized. Hence N 0.

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Molecular Geometry Ck 12 Foundation

How To Draw The Lewis Structure Of Pcl5 Phosphorus Pentachloride Youtube
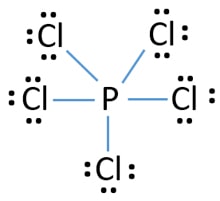
Pcl5 Phosphorus Pentachloride Lewis Structure

Pcl5 Lewis Structure How To Draw The Lewis Structure For Pcl5 Youtube
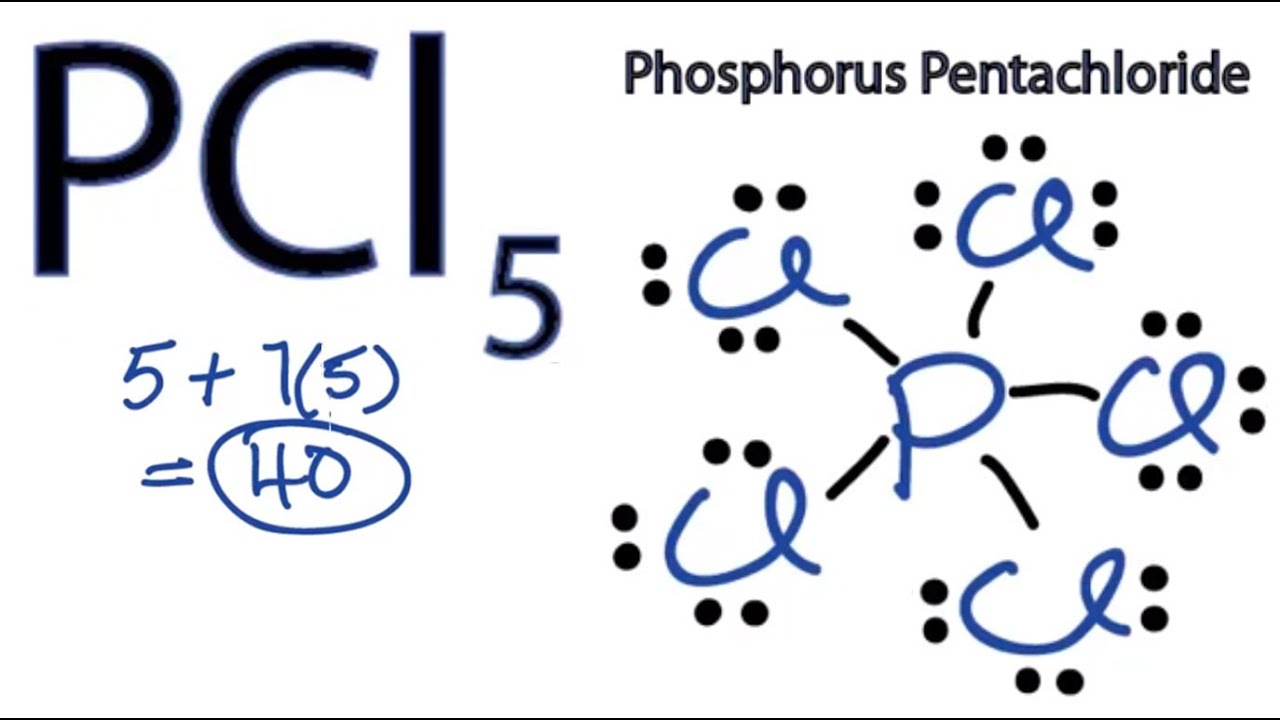
Pcl5 Lewis Structure How To Draw The Lewis Structure For Pcl5 Youtube
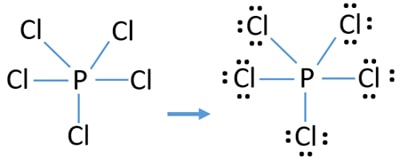
Pcl5 Phosphorus Pentachloride Lewis Structure

Chapter 11 Molecular Geometry Polarity Of Molecules And Advanced Bonding Theory
0 comments
Post a Comment